The Plastic Surgery Channel / Breast Videos / Breast Implants Videos
Breast Implants Videos
-
PSC Roundtable
Surgical Algorithm for Management of Implant Exchange with Textured Implants
On this The Aesthetic Society Roundtable, board certified plastic surgeons and breast augmentation experts Dr. William P. Adams Jr. of Dallas, Texas, Dr. Brad Calobrace of Louisville, Kentucky, Dr....
William P. Adams, Jr., MD August 31, 2020 -
PSC No Spin LIVE
Sientra TECHnews Breast Augmentation: Episode 2
On the second episode of Sientra TECHnews Breast Augmentation, Sientra’s Blaine Hamilton is joined by veteran plastic surgeons Dr. William P. Adams Jr., Dr. Andrew Wolfe, Dr. Michael Schwartz,...
PSC May 15, 2020 -
PSC No Spin LIVE
No Spin Live Episode 93 – Board Certified Plastic Surgeons Respond to COVID-19
On Episode 93 of No Spin Live, board certified plastic surgeons Dr. Dustin Reid, Dr. Ashley Reid, and Dr. Ned Snyder IV of Austin, Dr. William P. Adams Jr....
William P. Adams, Jr., MD April 10, 2020 -
PSC Uncut
The Lifespan of Breast Implants
One of the first questions that many breast augmentation patients ask during their initial consultation is whether or not they will need to replace their implants in 10 years....
Katherine Stuart February 28, 2020 -
Deep Dive
PSC Deep Dive – Concerns About Heavy Metals in Breast Implants
Amplified by the growth of social and digital media, the concern of heavy metal toxicity has re-surfaced again over the past 2 years. In particular, breast implant patients (including...
Katherine Stuart January 21, 2020 -
PSC No Spin LIVE
No Spin Live Episode 82 – Breast Implant Removal Warning
In another informative and engaging No Spin Live, board certified plastic surgeons Dr. Jason Pozner, Dr. Mark D. Epstein, and Dr. William P. Adams Jr. discuss textured breast implants...
William P. Adams, Jr., MD January 16, 2020 -
The Surgeon Minute
Risk Reduction for Breast Implant-Associated ALCL
Many breast augmentation patients are alarmed by Allergan’s recent recall of their BIOCELL textured breast implants, due to their association with Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL), and...
Katherine Stuart January 13, 2020 -
PSC No Spin LIVE
No Spin Live Episode 79 – Australia’s Response to BIA-ALCL and Textured Implants
The recent announcement by the Therapeutic Goods Administration (TGA), a Division of the Australian Department of Health, to temporarily suspend the sale of eight different textured implants for the...
Katherine Stuart October 31, 2019 -
PSC No Spin LIVE
No Spin Live Episode 64 – The Recent FDA Committee Meeting on Breast Devices
No Spin Live host Dr. William P. Adams Jr. of Dallas welcomes board certified plastic surgeons Dr. Jason Pozner of Boca Raton, Florida, Dr. Melinda Haws of Nashville, and...
William P. Adams, Jr., MD May 3, 2019 -
The Surgeon Minute
Making The Most of Your Breast Augmentation Revision Consultation
As with any operation, a patient hopes for a perfect outcome. Sometimes, however, there is a need, or even just a desire, to repeat a surgical procedure. It can...
Dawn Tongish January 16, 2019 -
The Surgeon Minute
Is a Breast Lift or a Breast Augmentation The Right Choice?
There are a lot of reasons why a woman’s breast appearance changes through the years – pregnancy, weight loss or gain, genetics and age can all play a role....
Dawn Tongish January 2, 2019 -
Deep Dive
Maximizing Breast Implant Patient Safety: Raising Awareness of BIA-ALCL Part I
In this special 3 part series of PSC Deep Dive host William P. Adams Jr. MD moderates a group discussion on Breast Implant Associated-Anaplastic Large Cell Lymphoma (BIA-ALCL) with...
Anne Meyer October 22, 2018 -
The Surgeon Minute
3 Reasons Women Have Breast Implants Removed
When a celebrity has breast implants removed, it makes the headlines. Victoria Beckham, Melissa Gilbert, Tori Spelling and Pamela Anderson are among the growing number of Hollywood stars who...
Anne Meyer July 19, 2018 -
The Surgeon Minute
Choosing the Right Breast Implants
It can seem daunting and overwhelming to wade through the vast number of options available for breast augmentation. Many patients find it nearly impossible to determine what is the...
Dawn Tongish May 16, 2018 -
Deep Dive
PSC Deep Dive – Breast Implant-Associated ALCL – Part II
PSC Deep Dive continues with the discussion regarding Breast Implant Associated-ALCL . Plastic surgeons Dr. Caroline Glicksman and Dr. Pat McGuire discuss the disease with Dr. Marshall Kadin, a...
William P. Adams, Jr., MD April 28, 2018 -
The Surgeon Minute
Breast Implants: How To Pick The Best Bust For You
Breast augmentation remains the most popular plastic surgery procedure. Every year hundreds of thousands of women opt to have the appearance of their breasts changed, usually enlarged with implants....
Dawn Tongish April 26, 2018 -
The Surgeon Minute
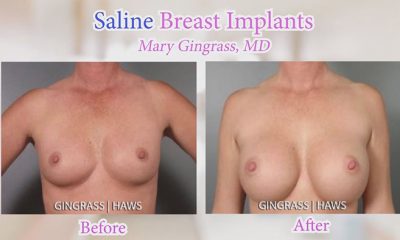
Breaking Down the Saline vs. Silicone Debate
Saline vs. silicone is kind of the “to be or not to be” of breast augmentations. It’s really one of the first (of many) decisions that breast augmentation patients...
Katherine Stuart April 24, 2018 -
PSC Uncut
The Front Line of Modern Breast Implants
Breast augmentation has never been better than it is today. Surgical technique has improved to the point that recovery for many is 24 hours long, with many patients able...
Theo Nyame, MD April 13, 2018 -
The Surgeon Minute
Saline to Silicone: Should You Make the Switch?
It’s a very common question surrounding breast surgery: Should I upgrade or switch to silicone implants, if I currently have saline implants? In general, most experts will say it...
Dawn Tongish April 9, 2018 -
The Surgeon Minute
New Implants Restore Volume in All the Right Places
For the past decade, surgeons have achieved great results with third and fourth generation silicone breast implants. Unfortunately, these implants tend to lose their shape once they are inside...
Anne Meyer January 24, 2018
-
BTS Stockholm LookLive 2024 – Mastopexy with Auto-augmentationLook-Live SurgeryJune 4, 2025
-
Inconspicuous Plastic SurgeryPSC RoundtableJune 4, 2025
-
Misconceptions About Breast ReconstructionPSC RoundtableJune 4, 2025
Breast Augmentation
-
BTS Stockholm LookLive 2024 – Mastopexy with Auto-augmentationLook-Live SurgeryJune 4, 2025
-
Inconspicuous Plastic SurgeryPSC RoundtableJune 4, 2025
-
Misconceptions About Breast ReconstructionPSC RoundtableJune 4, 2025


















Facebook
Twitter
Instagram
YouTube
RSS