Allergan recently issued a global recall of their BIOCELL textured breast implants and tissue expanders in response to the FDA’s warning of the potential risk between textured implants and patients developing BIA-ALCL, a rare form of large cell lymphoma. Dr. William P. Adams Jr. of Dallas and the No Spin Live panel of experts, Dr. Adam Hamawy of Princeton, NJ, Dr. Jason Pozner of Boca Raton, and Dr. Richard Baxter of Seattle discuss how this recall has affected their practice and what patients need to know about it.
Know Your Implants
Right after the recall, Dr. Pozner’s phone rang off the hook. Most of his patients did not keep the implant card that explained exactly what type of implant was placed during their initial breast augmentation. They were understandably scared, so the last few weeks have been spent educating patients on their implants. Luckily for Dr. Pozner, “I put zero of those implants in any of my patients so I didn’t have to worry about having to remove them or questioning any of my patients.” Still, he has seen a couple of new patients who have the BIOCELL implants for consultations and he did take out a pair the other day. The patient had developed capsular contracture, so the implants needed to come out regardless.
Dr. Pozner is finding that since the FDA released its warning, patients are doing their homework. They come in to the office asking about ALCL and their risks, which is always a good thing. “My preference has always been smooth implants and the rate is either really low or zero,” so the textured implant debate has not really affected his practice.
Patients Confused by Term “Recall”
Dr. Adams has found that patients are really confused by the term “recall.” This is not a recall like in the auto industry where you have to bring your car back in and have the part replaced immediately. “This is a withdrawal of unused product and further sales of BIOCELL ,” he explains. In their statement, the FDA was very clear about the fact that they do not recommend that asymptomatic women have their implants removed or replaced.
Dr. Hamawy has had a similar experience: patients are calling the office because they’re not sure what to do. In the last few years, he has mostly placed smooth implants, so “it’s just reassuring them and telling them what they have and then informing them that they really aren’t at risk,” he explains.
Patients Need to Be Reassured & Educated
Dr. Baxter, on the other hand, does have a small number that he’s put in, “because I do think there are indications for using shaped implants sometimes.” His staff got in touch with all of those patients. He’s also received many calls from other patients who are understandably upset. “They’re scared,” he shares. “They really need good information and they’re frustrated that they didn’t hear about this sooner.”
Despite the fact that the panel of experts have been discussing this matter for a number of years, the information has only recently trickled down to the mainstream media. Dr. Baxter’s approach has been to offer his patients reassurance and explain to them that the risk of developing ALCL is extremely low. Furthermore, if you know what to watch for and catch it early, ALCL is also highly treatable.
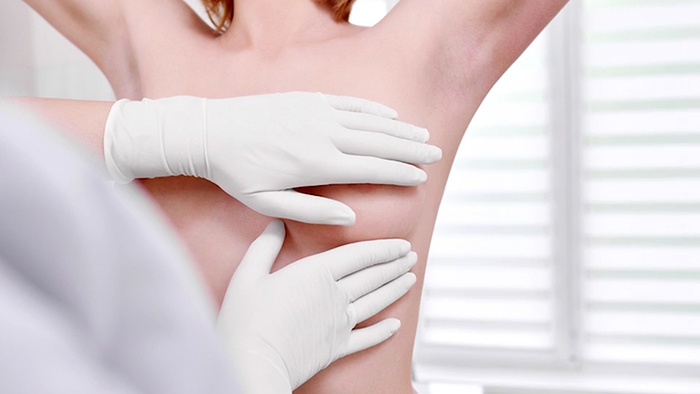
Potential Warning Signs of ALCL
Allergan has offered a set of smooth implants to any patient who wishes to have her BIOCELL implants exchanged. If you develop any of the following symptoms, schedule an appointment to be assessed in person by your board certified plastic surgeon:
- enlargement or swelling of the breast
- firmness in the breast
- pain or tenderness
- unexplained rash
All of the NSL experts offer their breast augmentation patients an annual check up to be evaluated. Take advantage of this! It’s the best way for both you and your surgeon to ensure that your implants remain healthy.
















Facebook
Twitter
Instagram
YouTube
RSS