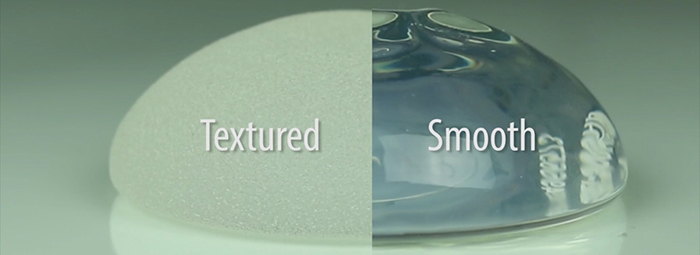
With Allergan’s recent recall of their BIOCELL textured breast implants from the global market, there has been a lot of media attention on breast implant-associated ALCL and what a patient needs to do to protect herself. The online information is often confusing, misleading and, sometimes, down right false. Worse, certain unscrupulous people are preying on patients’ fear, telling them that they need surgery when they do not.
Board certified plastic surgeon Dr. William P. Adams Jr. of Dallas discusses these online misnomers with his colleagues Dr. Pat McGuire of St. Louis, MO, Dr. Mindy Haws of Nashville, TX, Dr. Caroline Glicksman of Sea Girt, NJ and Dr. Anand Deva of Sydney, Australia in order to help patients separate fact from fiction.
Is Capsulectomy Necessary with Implant Removal?
On July 24, Allergan issued a voluntary recall of their BIOCELL textured breast implants and tissue expanders. Dr. McGuire’s phone immediately starting ringing off the hook with calls from both patients and other surgeons who were confused as to what to do. It’s important to first understand that the FDA does not recommend that a patient with a BIOCELL or textured implant have her implant removed if she is not having issues. Nevertheless, some patients make the personal decision that they would like to remove their textured breast implants.
Dr. McGuire notes that patients have been trained to ask whether or not the capsule should be removed, too. “There really isn’t any long term studies on this,” explains Dr. McGuire. There have been cases where a patient had her implants removed, left the capsule, and then developed ALCL. That said, all of these patients already had symptoms at the time of the removal. Her recommendation to patients is if they want their implants out and the capsule can be safely removed then yes, she performs a capuslectomy.
Safety is the Key
This is not like a recall on a car part, explains Dr. Haws. If you are not experiencing any issues with your implants, you do not need to have them removed. However, if you are uncomfortable with the risk, or it’s been 10-15 years since your original surgery, it may be time to exchange your implants. At the least, it’s time to follow-up with your surgeon. And yes, if the surgeon can safely remove the capsule, then they will. Sometimes, the capsule is tissue paper-thin and removing it can pose a danger, such as puncturing a lung or harming surrounding muscles. If it’s not worth the risk, a great surgeon will not do it.
Balance Risk & Benefit
Unlike here in the United States, there are a number of different textured implants on the market in Australia. Dr. Deva estimates that 95% of the implants going into patients in his country are textured. He is experiencing the same issues with patient confusion as Dr. McGuire and Dr. Haws. Medical recalls come in different grades and this one is simply a removal of merchandise from the shelves and warehouses. It does not mean a mandatory replacement. So, if a patient is asymptomatic, there is no reason to remove her implants.
“Our jobs as surgeons is to balance risk and benefit,” he explains. There is often a bigger risk of removing an implant that is not causing a problem than there is benefit to the patient. It’s the job of the surgeon to educate the patient. He understands that patients are scared. He tells them to come into to his office 100 times a year if that reassures them, but he can’t justify subjecting a patient to an unnecessary operation. Performing a complete capsulectomy is probably the only way to reduce the risk of ALCL, and, as discussed, it can be a risky surgery.
Language Around Texture Needs to Change
It is known that the more textured an implant, the higher the risk of developing ALCL. All of our experts agree that the language around texture has to change. In Australia, Dr. Deva and his colleagues are beginning to use a numeric scale. For example, the highest level is Grade 4, which is a polyurethane covered implant, and carries the highest known risk in Australia of about 1 in 2,000 of developing ALCL. Then there is Grade 3, such as BIOCELL, with a risk of 1 or 2 in 3,000 and Grade 2 with a risk of 1 in 80,000. Grade 1 is a smooth implant with no known risk of ALCL.
With all of this negative attention, patients and surgeons have forgotten about the benefits of textured implants. They are great options for difficult cases, such as a patient with tuberous breasts, a breast reconstruction patient following mastectomy, or in an aesthetic patient who does not want a breast lift. “So, let’s not throw the baby out with the bath water,” says Dr. Deva.
Speaking to the Patient in a Language She Understands
Part of the problem when dealing with patient anxiety is “how patients understand risk,” explains Dr. Glicksman. There is something called numeracy, which is a patient’s conception or understanding of what numbers mean. Unfortunately, it’s not the same for every patient. Dr. Glicksman recently saw 8 patients in one day who had come in about the recall. She tried a number of different approaches with numbers to communicate how low the risk of ALCL is, such as the fact that every woman has a 1 in 8 chance of developing breast cancer. This is a 12% risk versus ALCL which has a risk factor of .003%. The problem is patients are scared; they don’t want to hear the numbers.
Every patient requires a different approach. Dr. Glicksman works hard to calm the patient and then explain the risks in a language that patient will understand. Oftentimes, the issue isn’t with the patient, but with a family member, such as a husband or child, who has read about ALCL online and thinks that the patient must have her implants removed. The patient and her plastic surgeon must consider the aesthetic implications of replacing her textured implant with a smooth implant. It may reduce your risk of ALCL, which is already extremely rare, but increase your risk of something else. It’s always about balancing the risk and the benefit for each individual patient.
Be Wary of Exploitation
With implant removal and ALCL, there is a lot of misinformation online. Many patients think that if they have a capuslectomy then it must be sent in for CD30, but this test is not a diagnostic for BIA-ALCL, nor is it indicated in the situation. With tissue such as a capsule, the pathologist can look under a microscope ,and if no abnormal lymphocyctes are present, then there is no reason to do a CD30.
The same is true of an “en bloc capsulectomy.” Whenever there is fear, there are always going to be people preying on that fear. Unscrupulous doctors are using shiny marketing to lure patients in for surgery at a cheap price. They tell them that they must remove their implants, even if they aren’t having any issues, and then promise an en bloc removal of the capsule, meaning that it is removed in one piece. First of all, it is impossible to promise this. And second, “Dr. Google is not going to predict what kind of surgery I am going to do on my patients,” says Dr. Glicksman. She is going to perform the safest surgery that meets the needs of the patient which is to address anything abnormal.
Informed Consent & Patient Education
Dr. Haws leads a task force for the aesthetic society on informed consent. This is something that needs to change. Again, it’s not a one size fits all. Informed consent needs to be in the language that the patient will understand, whether that’s video, verbal or written. In Australia, Dr. Deva and his colleagues refer to it as informed educated consent. Dr. Adams loves this term as he feels that patient education and informed consent are intertwined. Patient must be educated in order to understand, otherwise, there will never be truly informed consent.
It really comes down to an issue of trust. The patients don’t trust the FDA, the implant manufacturers, nor the physicians, and are often misplacing their trust in people who are pandering to their fear. “I think it’s our job to reestablish trust,” concludes Dr. Deva.



















Facebook
Twitter
Instagram
YouTube
RSS